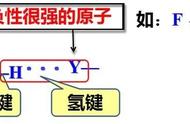
8-羟基喹啉团簇间的分子间氢键。图片来源:Science [12]
2013年,中国科学院国家纳米科学中心的裘晓辉研究员课题组在Science 上发表论文(上图)[13],他们利用原子力显微镜技术,观测到分子间氢键和配位键相互作用,在国际上首次实现了分子间作用的直接成像。分子间氢键的“照片”被同年的Nature 评为年度最震撼的图片之一(下图)[14]。

图片来源:Nature News [14]。
氢键无处不在。它是有机反应的幕后推手,是催化剂的设计指南,是生物体内的平行世界,是化学吸附的传感器,是光谱红移蓝移的指南针,是材料组装最后的机理解释。关于氢键的研究还在继续,且永无止境。如果你相信弱作用力的说法和一条细线,那么就举杯庆祝氢键诞生108周年;如果你更欣赏Latimer和Rodebush对氢键明确且丰富的描述,那么,就举杯庆祝氢键百岁快乐!
参考文献:
[1] Why does ice float in water?
https://ed.ted.com/lessons/why-does-ice-float-in-water-george-zaidan-and-charles-morton
[2] Gibb B. C. The centenary (maybe) of the hydrogen bond. Nat. Chem., 2020, 12, 665-667. DOI: 10.1038/s41557-020-0524-2
https://www.nature.com/articles/s41557-020-0524-2
[3] https://en.m.wikipedia.org/wiki/Hydrogen_bond
[4] Pauling L. J. Am. Chem. Soc., 1935, 57, 2680-2684. DOI: 10.1021/ja01315a102
https://pubs.acs.org/doi/abs/10.1021/ja01315a102
[5] Latimer W. M. & Rodebush W. H. Polarity and Ionization from the Standpoint of the Lewis Theory of Valence. J. Am. Chem. Soc., 1920, 42, 1419-1433. DOI: 10.1021/ja01452a015
https://pubs.acs.org/doi/abs/10.1021/ja01452a015
[6] Arunan E., Desiraju G. R., Klein R. A., et al. Defining the Hydrogen Bond: An Account (IUPAC Technical Report). Pure and Applied Chemistry, 2011, 83, 1619-1636. DOI: 10.1515/ci.2011.33.5.25c
https://www.degruyter.com/view/journals/ci/33/5/article-p25.xml
[7] Moore T. S. & Winmill, T. F. J. Chem. Soc. Trans., 1912, 101, 1635-1676. DOI: 10.1039/CT9120101635
https://pubs.rsc.org/en/content/articlelanding/1912/CT/CT9120101635#!divAbstract
[8] Langmuir I. J. Am. Chem. Soc., 1919, 41, 868-934. DOI: 10.1021/ja02227a002
https://pubs.acs.org/doi/10.1021/ja02227a002
[9] Lewis G. N. J. Am. Chem. Soc., 1916, 38, 762-785. DOI: 10.1021/ja02261a002
https://pubs.acs.org/doi/abs/10.1021/ja02261a002
[10] Huggins M. L. Hydrogen bridges in ice and liquid water. J. Phys. Chem., 1936, 40, 723-731. DOI: 10.1021/j150375a004
https://pubs.acs.org/doi/abs/10.1021/j150375a004
[11] Watson J. D. & Crick F. H. C. Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid. Nature, 1953, 171, 737-738.
https://www.nature.com/articles/171737a0
[12] Kitaura K. & Morokuma K. Int. J. Quantum Chem., 1976, 10, 325-340. DOI: 10.1002/qua.560100211
https://onlinelibrary.wiley.com/doi/abs/10.1002/qua.560100211
[13] Zhang J., et al. Real-Space Identification of Intermolecular Bonding with Atomic Force Microscopy. Science, 2013, 342, 611-614. DOI: 10.1126/science.1242603
https://science.sciencemag.org/content/342/6158/611
[14] 365 days: Images of the year
https://www.nature.com/news/365-days-images-of-the-year-1.14303
(本文由小希供稿)