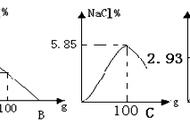
1、铁和硫磺:________________________________________________;
Fe S==(加热)FeS
2、铜和硫磺:________________________________________________;
2Cu S==(加热)Cu2S
3、硫磺和氧气:______________________________________________;
S O2==(点燃)SO2
4、硫磺和汞:________________________________________________;
Hg S==HgS
5、硫磺溶于热的浓氢氧化钠:________________________________________________;
3S 6NaOH==(加热)2Na2S Na2SO3 3H2O
6、硫化氢和少量氢氧化钠:__________________________________________________;
H2S NaOH==NaHS H2O
7、硫化氢和足量氢氧化钠:__________________________________________________;
H2S 2NaOH==Na2S 2H2O
8、实验室制备硫化氢:_____________________________________________________;
FeS H2SO4==FeSO4 H2S↑
9、硫酸亚铁和硫化钠:_____________________________________________________;
FeSO4 Na2S==FeS↓ Na2SO4
10、硫化氢和硫酸铜:______________________________________________________;
H2S CuSO4==CuS↓ H2SO4
11、检验空气中是否含有硫化氢:_____________________________________________;
(硫化氢和硝酸铅)
H2S Pb(NO3)2==PbS↓ 2HNO3
12、硫化氢完全燃烧:_____________________________________________________;
2H2S 3O2==(点燃)2H2O 2SO2
13、硫化氢不完全燃烧:___________________________________________________;
2H2S O2==(点燃)2H2O 2S
14、硫化氢在空气中被氧化:_______________________________________________;
2H2S O2==2H2O 2S
15、硫化氢和氯气(溴水,碘水):__________________________________________;
H2S Cl2==2HCl S
16、硫化氢和次氯酸钠:__________________________________________________;
H2S NaClO==NaCl S↓ H2O
17、硫化氢和二氧化硫:__________________________________________________;
2H2S SO2==3S 2H2O
18、硫化钠 亚硫酸钠 盐酸:______________________________________________;
2Na2S Na2SO3 6HCl==3S↓ 6NaCl 3H2O
19、二氧化硫 澄清石灰水:_______________________________________________;
SO2 Ca(OH)2==CaSO3↓ H2O
20、过量的二氧化硫 氢氧化钙:___________________________________________;
2SO2 Ca(OH)2==Ca(HSO3)2
21、盐酸 亚硫酸钙:____________________________________________________;
2HCl CaSO3==CaCl2 SO2↑ H2O
22、实验室制二氧化硫:_________________________________________________;
Na2SO3 H2SO4(浓)==Na2SO4 SO2↑ H2O
23、二氧化硫的催化氧化:_______________________________________________;
2SO2 O2==(可逆,催化剂,加热)2SO3 Q
24、亚硫酸钠在空气中氧化:_______________________________________________;
2Na2SO3 O2==2Na2SO4
25、二氧化硫和氯气溶于水(溴单质、碘单质):______________________________________;
SO2 Cl2 2H2O==H2SO4 2HCl
26、二氧化硫 次氯酸钠:_________________________________________________;
SO2 NaClO H2O==H2SO4 NaCl
27、铜和浓硫酸:_________________________________________________________;
Cu 2H2SO4(浓)==(加热)CuSO4 SO2↑ 2H2O
28、碳和浓硫酸:_________________________________________________________;
C 2H2SO4(浓)==(加热)CO2↑ 2SO2↑ 2H2O
29、胆矾和浓硫酸:______________________________________________________;
CuSO4·5H2O==(浓硫酸)CuSO4 5H2O
30、蔗糖和浓硫酸:______________________________________________________;
C12H22O11==(浓硫酸)12C 11H2O
31、碘化氢(溴化氢)和浓硫酸:________________________________________________;
2HI H2SO4(浓)==I2 SO2 2H2O
32、硫化氢和浓硫酸:____________________________________________________;
H2S H2SO4(浓)==S↓ SO2 2H2O
33、沸腾炉中的反应(工业制硫酸第一步):_________________________________________;
4FeS2 11O2==(煅烧)2Fe2O3 8SO2
,