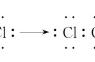1. Bonding of the carbon atom. 碳原子的结合。
Electronic structure of the carbon atom is 1s2 2s2 2px1 2py1.
碳原子的电子结构是1s2 2s2 2px1 2py1。
The outer electrons are located in orbitals (volumes of electron probability) having the following shapes 外层电子位于具有以下形状的轨道(电子概率的体积)中:

Carbon is unable to take part in ionic bonding since it is not energetically possible to form either C4 or C4-. It must therefore bond covalently by sharing electrons.
To achieve its maximum valency of 4. The 2s electrons must become uncoupled to give the electronic structure: 2s1 2px1 2py1
碳不能参与离子键,因为它在能量上不可能形成C4 或C4-。因此,它必须通过分享电子来进行共价键。
为了达到4的最大化合价,2S电子必须解耦,以获得电子结构:2S1 2px1 2py1

During bonding the 2s and 2p orbitals blend together to form four identical orbitals. This process is known as hybridisation.
在结合过程中,2S和2P轨道混合在一起,形成四个相同的轨道。这个过程被称为杂化。

2. Reactions of covalent bonds 共价键的反应
For any organic reaction to take place, covalent bonds must be broken. The factors influencing bond breaking are 要发生任何有机反应,必须打破共价键。影响断键的因素有:
a) Kinetic factors 动力因素
For bond breaking to occur, the molecules must possess a certain amount of energy (activation energy). Sufficient energy may be obtained through collisions with other molecules and transfer of kinetic energy to the bond.
Increasing the temperature of the reaction increases the number of molecules with energies in excess of the activation energy, a rise of about 10oC doubles the rate of reaction.
为使断键发生,分子必须拥有一定的能量(活化能)。足够的能量可以通过与其他分子的碰撞和动能向键的转移获得。
提高反应的温度会增加能量超过活化能的分子数量,上升约10oC,反应速度就会增加一倍。