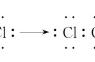b) Equilibrium of reactions 反应的平衡性
The reaction must have a favourable equilibrium constant.
该反应必须有一个有利的平衡常数。
3. Mechanism of bond breaking and making 断裂和生成键的机制
A covalent bond can be broken in two ways 一个共价键可以通过两种方式断裂:
a) Homolytic fission 同质裂变
- A:B → Ao Bo
Produces free radicals which have an unpaired electron and are very reactive.
产生自由基,自由基有一个未配对的电子,非常活跃。
b) Heterolytic fission 异质裂变
- A:B → A B-
- A:B → A- B
Produces ions.
产生离子。
4. Bond polarity 键的极性
Many organic molecules possess a dipole moment, due to an unequal distribution of electrons in the molecule. This occurs because some atoms tend to attractor repel electrons.
许多有机分子拥有偶极矩,这是由于分子中的电子分布不均。出现这种情况是因为一些原子倾向于吸引或排斥电子。
Consider the molecule, CH3Cl.
考虑一下分子, CH3Cl
Chlorine has a greater share of the electrons due to the electronegativity it possesses 由于氯气所具有的电负性,它拥有更大的电子份额:

This kind of bond polarisation is known as the inductive effect.
这种键的极化被称为感应效应。
The inductive effect is the power of an atom or group of atoms to attract electrons compared to the power of a hydrogen atom.
感应效应是指与氢原子的力量相比,一个原子或一组原子吸引电子的力量。
NO2 > Cl > I > NH3 > C-H < CH3 C2H5 < (CH3)2CH

Groups which are attracting electrons have a negative inductive (-I) effect. They give rise to electron deficient atoms Cδ .
Groups which are electron repelling have a positive inductive ( I) effect. They give rise to electron rich carbon atoms Cδ-.
吸引电子的基团具有负的感应作用(-I)。它们会产生缺电子的原子Cδ 。
驱赶电子的基团具有正的感应作用( I)。它们会产生富含电子的碳原子Cδ-。

Organic reagents can be classified as either 有机试剂可分为以下两类:
1. Nucleophiles: Attack centres of low electron density (nucleus loving). They possess a lone pair of electrons and are usually negatively charged.
1. 嗜核者。攻击电子密度低的中心(爱护核子)。它们拥有一对孤独的电子,通常带负电。
Examples include: H2O, ROH, OH-, RO-, Br-, NH3, RNH2, CN-.
2. Electrophiles: Attack centres of high electron density (electron loving) The are capable of accepting a lone pair of electrons and are usually positively charged.
2. 亲电体。高电子密度的攻击中心(热爱电子) 能够接受孤对电子,通常带正电。
Examples include: H , Br , R-N=N, CN-.
Functional groups 功能团The properties of an organic molecule are predominately determined by the properties of the functional group in that compound. Functional groups are atoms or combinations of atoms such as OH-, -COOH.
一个有机分子的特性主要由该化合物中的官能团的特性决定。官能团是原子或原子的组合,如OH-、-COOH。
Once the properties of the functional groups are known then the properties of any molecule containing a functional group maybe predicted.
一旦知道了官能团的特性,就可以预测任何含有官能团的分子的特性。
The common functional groups are listed below: